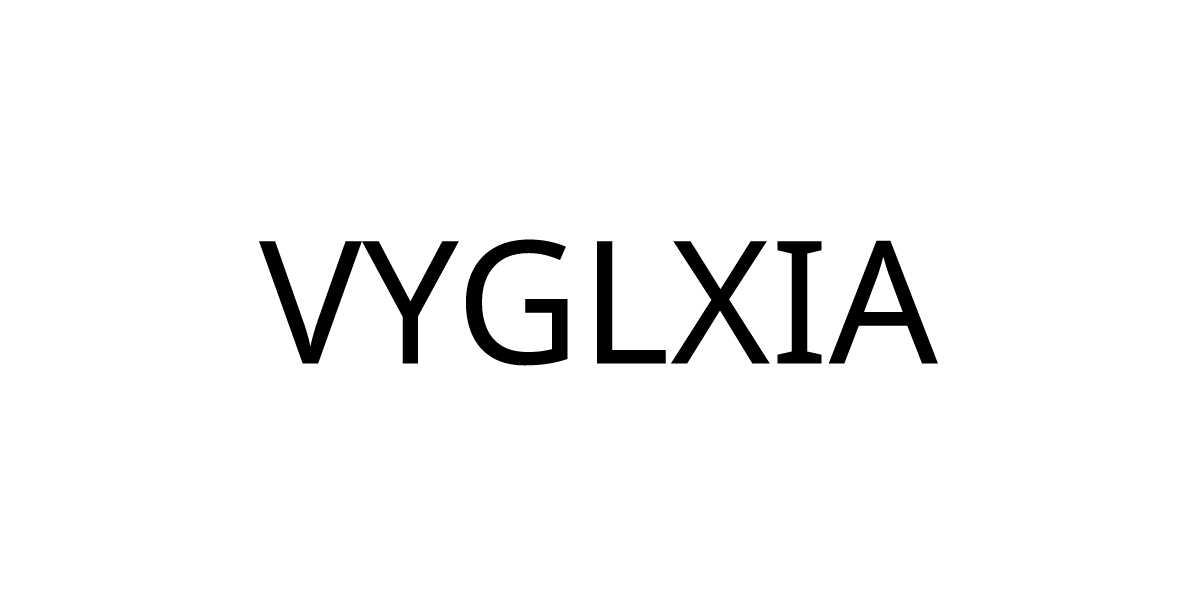Canada
Services
Categories
Trademarks
Protect your brand today
Register your Trademark
Post Filing Services
Trademark Protection
Patents
File a patent today
Patent your Inventions with
Copyrights
Safeguard your creative ideas
Register your creative ideas with
Start a Copyright Application
Business
Incorporate your business today
'Incorporate your business with
Start a Business
Legal Forms for Business
Resources
Support
C$
C$CAD
$USD
Call
Connect with us for legal assistance
(+1) 587 680-0257Email
Need help? Drop us an email
Email UsWhatsapp
Need a quick help? Leave us a message
Text Us